

This fluid offers the free choice among nearly all material data libraries available in EBSILONProfessional.
It is also possible to select several libraries for one stream. Please take note that in this case each library is calculated separately on its own. You can imagine this case as separate branches, isolated from each other by a flexible impermeable membrane. Between the individual branch streams, an equalization of pressure and temperature takes place, but not an exchange of material.
If a mixture calculation is to be performed, all materials to be mixed have to be included in the same material data library.
There are plenty of setting and combining options. However, not all of these make sense from the point of view of physics. It has to be noted that there are combinations and value ranges in which the material data libraries do not offer a solution. Therefore special care has to be taken when using the universal fluid. It is recommended to check on a small scale if the respective calculation is possible before modeling large cycles.
For the universal fluid, the temperature has to be determined recursively from the enthalpy. This may lead to problems in case of 2-phase states.
The material data libraries to be used have to be specified in the specification table first (column named "Library"). Then, the respective desired composition and other parameters are to be entered in the column “Specification”. The composition to be entered here refers to the mass fractions (or mole fractions with (right mouse button)) within the branch stream that is calculated by the respective library.
It depends on the respective library which materials are available and which additional input parameters are needed.
If a library is changed for the "Universal fluid" line type, the composition is now retained (of course only for substance components that are available in the new library).
| Material Data Library available in streams of type "Universal Fluid" | Substance name | Chemical Formula | Author | Available in streams of type 2-phase-fluid | Available also in streams of type |
|
One Material Libraries
|
|||||
| IF97 (IAPWS actual water steam table) | Water and Steam | H2O | KCE ThermoFluidProperties |
x |
Water, Steam |
| IF97 (IAPWS actual water steam table, SBTL) | Water and Steam | H2O | KCE ThermoFluidProperties | x | Water, Steam |
| IFC-67 (water steam table from 1967) | Water and Steam | H2O | KCE ThermoFluidProperties | x | Water, Steam |
| Lib-AmWa | Ammonia water mixture | NH3 / H2O | KCE ThermoFluidProperties | Binary mixture | |
| Lib-C10H22 | Decane | C10H22 | KCE ThermoFluidProperties | x | |
| Lib-C2H5OH | Ethanol | C2H5OH | KCE ThermoFluidProperties | x | |
| Lib-C3H6O | Acetone | C3H6O | KCE ThermoFluidProperties | x | |
| Lib-C5H10 | Cyclopentane | C5H10 | KCE ThermoFluidProperties | x | |
| Lib-C5H12 Iso | Isopentane | C5H12 | KCE ThermoFluidProperties | x | |
| Lib-C5H12 Neo | Neopentane | C5H12 | KCE ThermoFluidProperties | x | |
| Lib-C6H14 | Isohexan 2-Methylpentane | C6H14 | KCE ThermoFluidProperties | x | |
| Lib-C7H8 | Toluene | C7H8 | KCE ThermoFluidProperties | x | |
| Lib-C9H20 | Nonane | C9H20 | KCE ThermoFluidProperties | x | |
| Lib-CH3OH | Methanol | CH3OH | KCE ThermoFluidProperties | x | |
| Lib-CO | Carbon monoxide | CO | x | ||
| Lib-CO2 | Carbon dioxide | CO2 | KCE ThermoFluidProperties | x | |
| Lib-COS | Carbonyl sulfide | COS | KCE ThermoFluidProperties | x | |
| Lib-D4 | Octamethylcyclotetrasiloxane (D4) | C8H24O4Si4 | KCE ThermoFluidProperties | x | Oil / Melt |
| Lib-D5 | Decamethylcyclopentasiloxane (D5) | C10H30O5Si5 | KCE ThermoFluidProperties | x | Oil / Melt |
| Lib-D6 | Dodecamethylcyclohexasiloxane (D6) | C12H36O6Si6 | KCE ThermoFluidProperties | x | Oil / Melt |
| Lib-H2 (normal-Hydrogen) | Hydrogen (normal) | H2 | KCE ThermoFluidProperties | x | |
| Lib-H2 (Para-Hydrogen) | Hydrogen (Para) | H2 | KCE ThermoFluidProperties | x | |
| Lib-H2S | Hydrogen sulfide | H2S | KCE ThermoFluidProperties | x | |
| Lib-HE | Helium | He | KCE ThermoFluidProperties | x | |
| Lib-HUAirXiw (humid air as ideal mixture of real gases, also below 0°C) | Dry-air water steam ice | Dry-air H2O | KCE ThermoFluidProperties | Humid air | |
| Lib-Ice | Ice, Water and Steam including melting and sublimation regions | H2O | KCE ThermoFluidProperties | x | |
| Lib-iso-Butan | Butane (iso-Butane) | C4H10 | KCE ThermoFluidProperties | x | |
| Lib-MD2M | Decamethyltetrasiloxane (MD2M) | C10H30Si4O3 | KCE ThermoFluidProperties | x | Oil / Melt |
| Lib-MD3M | Dodecamethylpentasiloxane (MD3M) | C12H36Si5O4 | KCE ThermoFluidProperties | x | Oil / Melt |
| Lib-MD4M | Tetradecamethylhexasiloxane (MD4M) | C14H42O5Si6 | KCE ThermoFluidProperties | x | Oil / Melt |
| Lib-MDM | Octamethyltrisiloxane (MDM) | C8H24Si3O2 | KCE ThermoFluidProperties | x | Oil / Melt |
| Lib-MM | Hexamethyldisiloxane (MM) | C6H18Si2O | KCE ThermoFluidProperties | x | Oil / Melt |
| Lib-N2 | Nitrogen | N2 | KCE ThermoFluidProperties | x | |
| Lib-N2O | Nitrogen oxide | N2O | KCE ThermoFluidProperties | x | |
| Lib-n-Butan | Butane (n-Butane) | C4H10 | KCE ThermoFluidProperties | x | |
| Lib-NH3 | Ammonia | NH3 | KCE ThermoFluidProperties | x | |
| Lib-Propan | Propane | C3H8 | KCE ThermoFluidProperties | x | |
| Lib-R134A (1,1,1,2-Tetrafluoroethane, CF3-CH2F) | 1,1,1,2-Tetraflouroethane (R-134a) | CF3CH2F C2H2F4 | KCE ThermoFluidProperties | x | |
| Lib-RealAir (78.12 mol% N2, 20.96 O2, 0.92 Ar) | Standard dry air - gas and liquid including two-phase mixture | N2 O2 Ar | KCE ThermoFluidProperties | x | |
| Lib-SaltWater | Sea Salt water mixture | H20 | Ebsilon / Universität Bremen | Saltwater | |
| Lib-SeaWa 2009 | Sea Salt water mixture | H20 | KCE ThermoFluidProperties | Saltwater | |
| Lib-SeaWa 2013 | Sea Salt water mixture | H20 | KCE ThermoFluidProperties | Saltwater | |
| Lib-SecRef-Ammonia | Ammonia water mixture | NH3 / H2O | KCE ThermoFluidProperties | Binary mixture | |
| Lib-SecRef-Calcium-Chloride | Calcium chloride water mixture | CaCl / H2O | KCE ThermoFluidProperties | Binary mixture | |
| Lib-SecRef-Ethanol | Ethanol water mixture | C2H5OH / H2O | KCE ThermoFluidProperties | Binary mixture | |
| Lib-SecRef-Ethylene-Glycol | Ethylene glycol water mixture | C2H6O2 / H2O | KCE ThermoFluidProperties | Binary mixture | |
| Lib-SecRef-Glycerol | Glycerol water mixture | C3H8O3 / H2O | KCE ThermoFluidProperties | Binary mixture | |
| Lib-SecRef-Lithium-Chloride | Lithium chloride water mixture | LiCl / H2O | KCE ThermoFluidProperties | Binary mixture | |
| Lib-SecRef-Magnesium-Chloride | Magnesium chloride water mixture | MgCl / H2O | KCE ThermoFluidProperties | Binary mixture | |
| Lib-SecRef-Methanol | Methanol water mixture | CH3OH / H2O | KCE ThermoFluidProperties | Binary mixture | |
| Lib-SecRef-Potassium-Acetate | Potassium acetate water mixture | C2H3KO2 / H2O | KCE ThermoFluidProperties | Binary mixture | |
| Lib-SecRef-Potassium-Carbonate | Potassium carbonate water mixture | K2CO3 / H2O | KCE ThermoFluidProperties | Binary mixture | |
| Lib-SecRef-Potassium-Formate | Potassium formate water mixture | CHKO2 / H2O | KCE ThermoFluidProperties | Binary mixture | |
| Lib-SecRef-Propylene-Glycol | Propylene glycol water mixture | C3H8O2 / H2O | KCE ThermoFluidProperties | Binary mixture | |
| Lib-SecRef-Sodium-Chloride | Sodium chloride water mixture | NaCl / H2O | KCE ThermoFluidProperties | Binary mixture | |
| Lib-SO2 | Sulfur dioxide | SO2 | KCE ThermoFluidProperties | x | |
| Lib-Sugar Solution | Water sugar mixture | Ebsilon | |||
| Lib-WaLi (Wasser / Lithiumbromid - mixture) | Lithium chloride water mixture | LiCl / H2O | KCE ThermoFluidProperties | Binary mixture | |
|
Libraries including more then one material, one (or more in case of a mixture) need to be selected
|
|||||
| Lib-FDBR (based on FDBR polynomials, for ideal gases, coal, oil) | 83 classical materials | Ebsilon / FDBR | Air, Flue gas, Crude gas, Gas, Oil, Coal, User-defined Fluid | ||
| Library VLMIX - Vapour-Liquid-Mixture | about 80 materials | ||||
| Refprop | about 180 materials | NIST, Gaithersburg | x | ||
| CoolProp | 123 materials | http://www.coolprop.org/ | |||
| CoolProp-incompressible aqueous solutions mass-defined | 35 mixtures | http://www.coolprop.org/ | |||
| CoolProp-incompressible aqueous solutions volume-defined | 13 mixtures | http://www.coolprop.org/ | |||
| CoolProp-incompressible-pure | 60 materials | http://www.coolprop.org/ | |||
| TREND | about 170 materials | Ruhr University Bochum | |||
| Thermo-Liquid (Oil / Melt) | 21 materials of stream type Oil/Melt | Ebsilon | Oil / Melt | ||
| Lib-HuGas (ideal mixture of real gases with dissociation) | Nitrogen Oxygen Argon Neon Carbon-dioxide Carbon-monoxide Water Sulfur-dioxide | N2 O2 Ar Ne CO CO2 H2O SO2 | KCE ThermoFluidProperties | ||
| Lib-NASA (same substances as in streams of classical type) | 83 materials | NASA | |||
| Lib-NASAfull (EbsScript Interface Unit System, type UniversalSubstanceEnum) | 2047 materials | NASA | NASA | ||
| Lib-IdGas (ideal gases according to VDI 4670 with dissociation) | Argon Neon Nitrogen Oxygen Carbon-monoxide Carbon-dioxide Water Sulfur-dioxide Dry-air N2-in-air | Ar Ne N2 O2 CO CO2 H2O SO2 Air Air-N2 | KCE ThermoFluidProperties | ||
| Lib-IdGasMix (ideal gases) |
Argon Neon Nitrogen Oxygen Carbon-monoxide Carbon-dioxide Water Sulfur-dioxide Dry-air N2-in-air Nitric-oxide Hydrogen-sulfide Hydroxyl Methanol Methane Ethane Ethene Propane Propene N-Butane Iso-Butane Benzene Hydrogen Helium Ammonia Fluorine |
Ar Ne N2 O2 CO CO2 H2O SO2 Air Air-N2 NO H2S OH CH3OH CH4 C2H6 C2H4 C3H8 C3H6 n-C4H10 iso-C4H10 C6H6 H2 He NH3 F2 | KCE ThermoFluidProperties | ||
| UserProps DLL |
For this purpose, the user can create a dll that performs the calculation of the material values. |
The interface is described in the file ‘user_props.h’ in the subfolder ‘<Ebsilon installation directory>\Data\Examples\user_props_dll’. |
All materials available in Ebsilon | ||
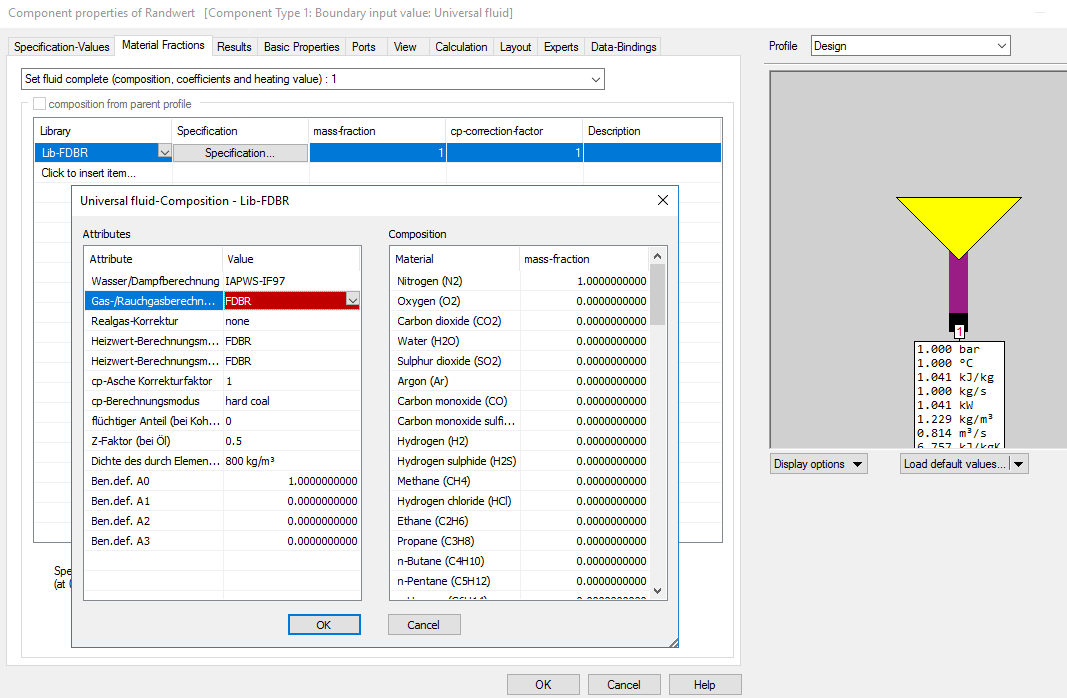
If the „Lib-FDBR“ is selected in the universal fluid, the calculation is performed according to FDBR (as used in any classical type stream) as ideal gas, according to the model settings for the gas- and water-formulation and the real gas correction.
But it is possible to select other gas- and water-formulations and real gas corrections as well. The default setting for these attributes is “according to model options”. In this case, when the model options are modified, these modification wills also affect all Lib-FDBR-universal fluids included in the model.
If you wish to generate pure FDBR-results, use the following settings together with component 1 or 33 for the attributes of the Lib-FDBR with the universal fluid:
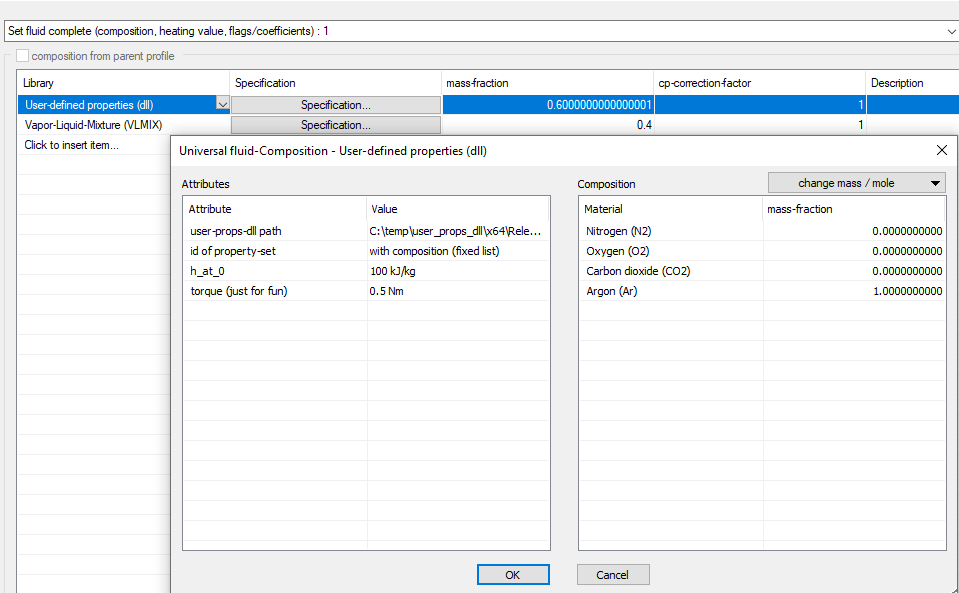
The “User-defined properties (Dll)” library (UserProps for short) is available for the universal fluid stream type. The user can create a Dll for this, which performs the calculation of the material values.
In contrast to the existing User2Phase Dll interface, UserProps allows the specification of compositions (substance value vectors) and additional attributes.
The interface is described in the file “user_props.h” in the subfolder “<Ebsilon installation directory>\Data\Examples\user_props_dll” and uses the C++ language.
There is also an example project there. It is recommended to use “Microsoft VisualStudio 2022” to create your own library.
Each “User-defined properties (dll)” entry of a universal fluid has the two standard attributes:
Additional attributes of the following types can be defined for each property set:
And a specification of which substances may occur (possibly none at all for pure substance models).
Further information about the functions and types of the interface can be found in the file “user_props.h”. The same directory also contains code for an example dll.
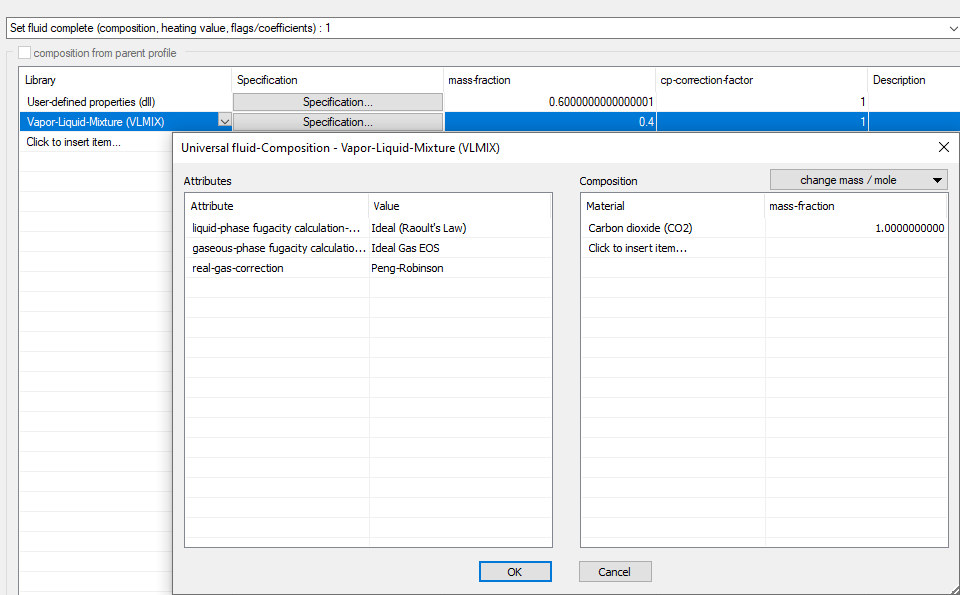
The VLMIX library extends the components available in FDBR with the ability to calculate vapour-liquid equilibria. The methods used are focussed on robust calculations with reasonable speed at the expense of reduced accuracy.
Furthermore, it is recommended to consider the limitations of the methods used as described below.
The thermal properties are calculated either with an ideal approach (pressure-independent) or with a cubic equation of state (Peng-Robinson, Redlich-Kwong, Soave-Redlich-Kwong).
In the ideal approach for deriving the enthalpy and entropy of the liquid phase, a regression function for the heat of vaporisation was used.
\[ H_{liq} = H_{gas} - dH_{vap} \]
\[ S_{liq} = S_{gas} - \frac{dH_{vap}}{T} \]
The mixing rules utilise the classic linear approach.
The following equation is valid for VLE:
\[ f_{i_{liq}} = f_{i_{gas}} \]
where \[f_{i_{phase}}\] is the fugacity of component i in the corresponding phase.
The fugacity of the liquid phase is calculated according to Raoult's law:
\[ f_{i_{liq}} = x_{i} * pvap_{i} \]
where \[x_{i}\] is the molar fraction of component i in the liquid phase and \[pvap_{i}\] is the gas pressure of pure component i at the current temperature.
It is assumed that the fugacity of the gas phase corresponds to the partial pressure defined in Dalton's law:
\[ f_{i_{vap}} = pres_{i} = y_{i} * pres \]
\[ \sum_{i} y_{i} = 1 \]
\[ \sum_{i} y_{i} * pres_{i} = pres \]
where \[y_{i}\] is the molar fraction, \[pres_{i}\] is the partial pressure of component i in the gas phase and \[pres\] is the actual pressure of the medium.
The gas pressure of each component is required to determine the VLE. For some components, data for an extended Antoine equation was available. For the remaining components, these were estimated as described below.
The Antoine equation for vapour pressure has the following form:
\[ ln (pvap_{i}) =A_{i} + \frac{B_{i}}{C_{i} + T} \]
Therefore, we need three data points to determine the parameters \[A_{i}, B_{i} \: and \: C_{i}\]
Candidates are the critical point, the triple point and the natural boiling point, which ensures that these points are exactly matched.
If we only have two data points, the following equation is used
\[ ln (pvap_{i}) =A_{i} + \frac{B_{i}}{T} \]
The described approach can only determine simple VLE with only one liquid phase. Even azeotropic behaviour (e.g. ethanol-water) cannot be described and mixtures close to the azeotropic point will deviate.
Since the VLE calculations use the Antoine equation for the vapour calculation, which itself is only valid up to the critical point, this approach describes the solubility of supercritical components in a liquid phase only poorly. In this case, an approach using Henry's law would be necessary, which is planned for a future release.
Furthermore, the heat of vaporisation for supercritical components is not defined when using the ideal gas formulation and the thermal properties of the liquid phase of such a component therefore deviate. Fortunately, the concentration of such components in the liquid phase is low.
At high pressure, when the compressibility factor \[Z_{i}\] is far from 1, the method used to calculate the volatilities of the gas phase leads to a larger error for the distribution of the composition between the individual phases.
Water and steam streams will not automatically switch to using LibIce, if you are in the corresponding range of state. The reason is that in the models usually represented with Ebsilon this is not desired anyway, but convergence problems can occur in the event of an automatic switchover, if temporarily values are assumed in the ice range in the course of the iteration. In particular, already the usual starting point of P=0.01 bar, H=10^-6 is barely in the two-phase range water/ice.
For the two-phase fluid, the entry “LibIce: Water” has been expanded to “LibIce: Water (3 phases)”. This allows modeling water in the entire range from -223.15 °C to 2,000°C. For temperatures up to 350°C, LibIce is activated, above this LibIf97.
The ‘User-defined properties (dll)’ library (UserProps for short) is available for the universal fluid line type. The user can create a dll for this, which performs the calculation of the material values.
In contrast to the existing User2Phase dll interface, UserProps allows the specification of compositions (substance value vectors) and additional attributes.
The interface is described in the file ‘user_props.h’ in the subfolder ‘<Ebsilon installation directory>\Data\Examples\user_props_dll’ and uses the C++ language. An example project can also be found there. It is recommended to use ‘Microsoft VisualStudio 2022’ to create your own library.
Each ‘User-defined properties (dll)’ entry of a universal fluid has the two standard attributes:
Additional attributes of the following types can be defined for each property set:
and a specification of which substances may occur (possibly none at all for pure substance models).
Further information about the functions and types of the interface can be found in the file ‘user_props.h’. The same directory also contains code for an example dll.